Search Results
Results for: 'Low Pco2'
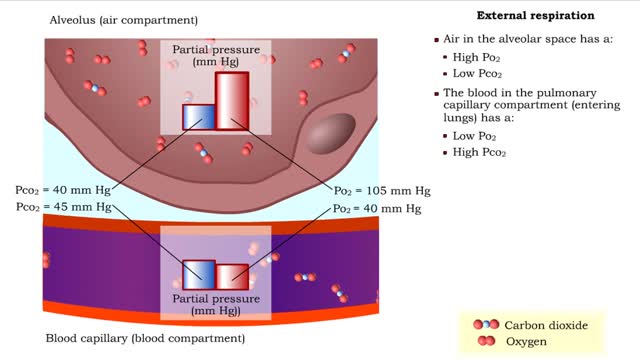
Gas exchange - partial pressure, locations, external and internal respiration
By: HWC, Views: 6808
▪ In a mixture, each individual gas exerts a pressure that is proportional to the concentration of that gas within the mixture. • This part of the total pressure is called a "partial pressure". • A gas moves along the part of the pressure gradient determined by its own concentration. ...
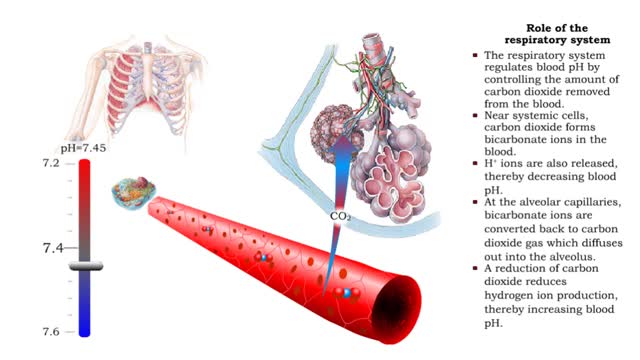
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 7166
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
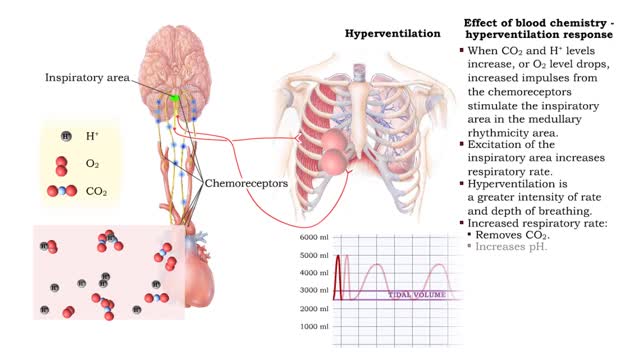
Effect of blood chemistry - stimuli, hyperventilation response and hypoventilation response
By: HWC, Views: 6428
• Respiratory rate is effected by changes in: • Blood pH. • Blood Pco2. • Blood P02. • Chemoreceptors in the central and peripheral nervous systems closely monitor the Fr, CO2 and 02 levels in blood. • Changes in frequency of impulses from Chemoreceptors affect respiratory r...
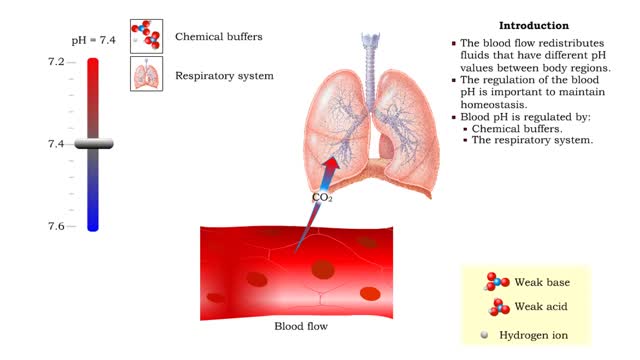
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 6526
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
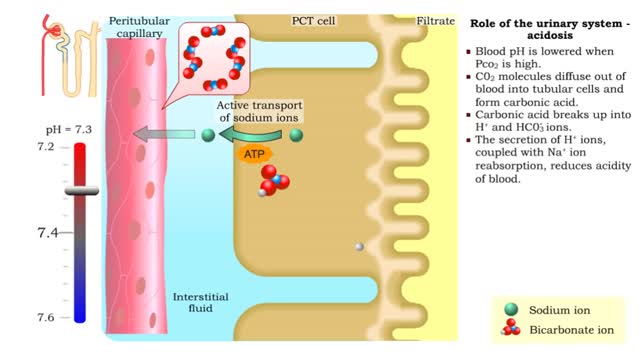
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 6875
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
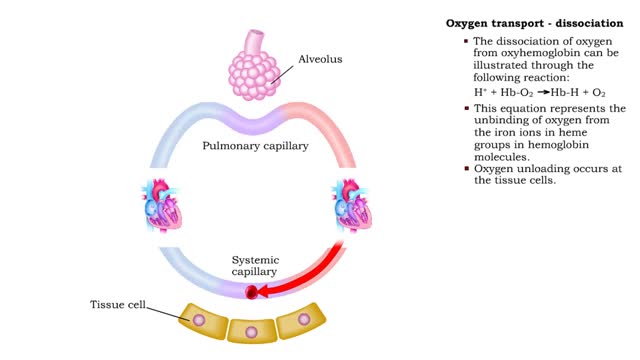
Oxygen transport: association and dissociation & Factors that affect hemoglobin's saturation with O2
By: HWC, Views: 6604
• The production of oxyhemoglobin can be illustrated through the following reaction: 02 + Hb-H --) Hb-02 + H+ • This equation represents the binding of oxygen to the iron ions in heme groups in hemoglobin molecules. • Oxygen binding or loading occurs at the lungs • The dissociatio...
Advertisement